Vanadium Redox Flow Battery “VRFB” 101
The Vanadium Redox Flow Battery
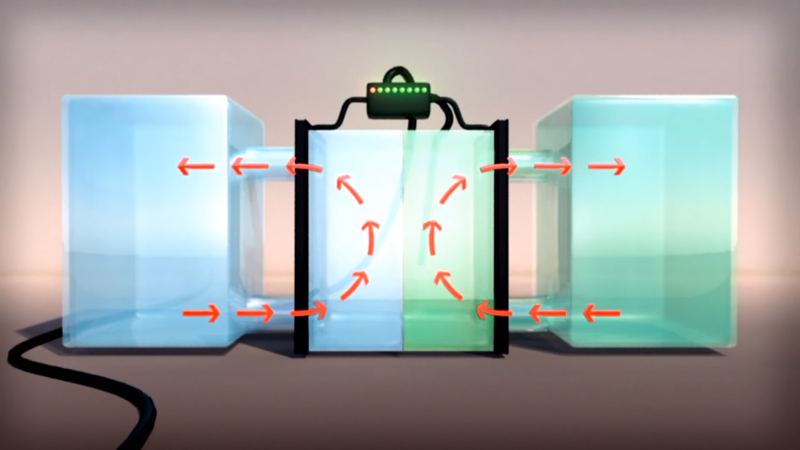
The VRFB is a type of rechargeable flow battery where rechargeability is provided by vanadium electrolyte (VE) dissolved in solution. The two tanks of Vanadium, one side containing V2+ and V3+ ions, the other side containing V4+ and V5+ ions, are separated by a thin proton exchange membrane. VRFBs consists of two tanks of vanadium electrolyte that flow adjacent to each other past a membrane and generate a charge by moving electrons back and forth during charging and discharging. This battery offers unlimited energy capacity simply by using larger electrolyte storage tanks. It can be left completely charged for long periods without losing capacity and maintenance is much simpler than other batteries. Pumps on both sides circulate the electrolyte.
The electron differential between the two cells generates electric power. Most batteries use two chemicals that change valence (or charge or redox state) and cross-contaminate and thus degrade over time. VRFBs utilize multiple valence states of vanadium as a single element to store and release charge. The VRFB has no cross-contamination like most batteries. The electrolyte in the catholyte and the anolyte consists of 100% vanadium ions. The ion-sensitive membrane separating both sides of the electrolyte tank allows only protons to pass. VRFBs are containerized, long duration, non-flammable, compact, reusable over infinite cycles, and last more than 20 years.
What are the advantages of Vanadium Redox Flow Batteries?
- VRFBs have a lifespan of 20+ years
- VRFBs offer immediate energy release
- VRFBs are suitable for grid connection or off-grid settings – ideal for renewable energy
- VRFBs can discharge 100%, without any damage to the battery
- VRFBs are non-flammable
- They ensure power and energy can be scaled independently
- Vanadium electrolyte can be re-used and does not need to be disposed of
- The batteries can be cycled more than once per day
- They use only one element in the electrolyte – V2O5
- VRFB energy storage guarantees uninterrupted power supply
How does a Vanadium Redox Flow battery (VRFB) work?
- A flow battery is charged and discharged by a reversible reduction-oxidation reaction between the two liquid vanadium electrolytes of the battery
- Unlike conventional batteries, electrolytes are stored in separated storage tanks, not in the power cell of the battery
- During operation, these electrolytes are pumped through a stack of power cells, in which an electrochemical reaction takes place and electricity is produced